|
|
 UF Researchers Seek Gene Therapies For Devastating BlindnessBy April Frawley Birdwell and John Pastor Josh Henderson was 16 when he noticed the vision in his left eye was getting blurry. A junior in high school who wrestled, played soccer and had just earned his driver license, Henderson had been wearing glasses for about a year and thought he might need a new prescription. But the blur became impenetrable. He had to give up driving and playing soccer. Video games became a struggle, too. Then, the same frightening progression started in his right eye.
Now 19, Henderson attends Santa Fe Community College. He knows Braille and can read some printed text with magnification, but it is slow, painstaking work. Henderson typifies the thousands of people every year who lose their eyesight to any of dozens of eye diseases caused by gene mutations or environmental factors. He is the kind of patient University of Florida scientists hope to help with new gene therapies that could replace broken genes with healthy ones to prevent blindness or restore sight. Although clinical treatments are still years away, UF researchers are making great progress, curing blindness in animals born blind, pinpointing problematic genes and finding model animals to help them get to the root of these disorders. Puppy Love“Bill, Bill, the dogs can see!” It was fall 2001, but William Hauswirth, a professor of ophthalmic molecular genetics with the UF Genetics Institute, remembers the day when he heard this news as if it were yesterday. He and scientists at Cornell University and the University of Pennsylvania had been experimenting with gene therapy to restore sight to three Briard puppies born with a genetic defect that causes blindness. Briards are a French sheepdog prone to a condition closely resembling Leber congenital amaurosis, or LCA, a blinding disease caused by mutations in a gene important for eye function. UF researchers developed and are leading proponents of using the apparently harmless adeno-associated virus, or AAV, as a vehicle for carrying corrective genes to different parts of the body. In this experiment, AAV would carry a corrective gene for LCA that would make a protein in the retina critical to translating light waves into nerve impulses that the brain interprets as images.
In late 2000, the Briard puppies had their right eyes treated with a single injection of thousands of copies of the corrective gene. Their left eyes were untreated as a control. The scientists planned to formally test eye function three months later, when the dogs were six months old. “Well before those tests, the researchers thought the dogs could see because they kept turning their heads to the right to use the eye that had been treated,” says Hauswirth. For the first time, scientists had given sight to a congenitally blind animal larger than a mouse, with eyes similar in size to the human eye. Five years have gone by since the Briard puppies — “Lancelot” was the breakout star, going on to shake paws with lawmakers on Capitol Hill — acquired sight. Now, UF researchers will find out whether the treatment works in people. Safety tests on human patients with congenital blindness are taking place this year at UF and the University of Pennsylvania. The human disease modeled by the dogs — Leber congenital amaurosis type 2, or LCA2 — affects about 2,000 people in the United States and is one of several incurable forms of blindness collectively known as retinitis pigmentosa, which afflict about 200,000 Americans. Newborns with LCA2 experience rapid degeneration of the retina, often leading to total vision loss by age 10. Only adults will participate in the first phase of the study, but infants and children will be examined for possible future treatment. “The idea of the therapy is simple,” said Hauswirth, the Rybaczki-Bullard Professor of Ophthalmic Molecular Genetics. “If your cells are missing a gene for a vital function, such as vision, the therapy is to replace that gene.” Gene therapy techniques also look promising to treat another form of blindness called juvenile retinoschisis, an incurable eye disease that affects about 4,000 Americans, mostly young boys. In a healthy eye, retinal cells secrete a protein called retinoschisin, or RS1, which acts like glue to connect the layers of the retina. Without it, the layers separate and tiny cysts form, obstructing vision. UF researchers injected AAV containing a healthy version of the human RS1 gene into the eyes of 15-day-old male mice, which, like boys with the disease, cannot produce retinoschisin. The condition in the mice was roughly equivalent to retinoschisis in a 10-year-old boy. After the treatment, researchers looked at the interior of the mice eyes with a laser ophthalmoscope and found cyst formation in the untreated eyes. But the treated eyes appeared healthy, even six months after the treatment. “We’ve been very successful in curing a disease in mice that has a direct copy in humans,” Hauswirth said. “It may take two to five years before we try this in human patients, but we feel based on success so far, we will be able to provide formal evidence for safety that will allow us to get treatment into the clinic.” Monkey ModelCayo Santiago is 45 acres of sunshine and palm trees, just a short boat ride from the southeast corner of Puerto Rico. Since 1938, the island has been home to a colony of rhesus monkeys — the same colony that helped identify a characteristic of a person’s blood known as the Rhesus, or Rh, factor.
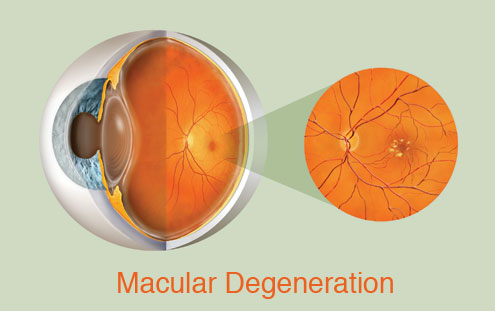
William Dawson, a UF professor of ophthalmology and physiology, has traveled to the island twice a year for 20 years to examine the monkeys’ eyes. In Puerto Rico and at UF, which keeps a small colony of the monkeys, Dawson and other researchers perform ophthalmologic exams, seeking clues to age-related macular degeneration, an eye disorder with both genetic and environmental causes that affects more than 6 million people in the United States. Dawson describes the pathology as a waste management problem. During the course of the disease, pieces of vascular tissue break off and build up in the eye. The damage occurs at the macula, which lies at the back of the retina and is responsible for producing sharp vision. This colony of monkeys is valuable for study because of inbreeding — about 70 percent of the older animals show signs of hereditary macular degeneration. In addition, the monkeys age three to four times faster than humans, making it easier for scientists to study the decline of the eye. Even more importantly, their eyes share the same complex structure as the human eye. Dawson used to slip images of rhesus monkey eyes into presentations for medical residents to see if they would notice the difference. But the similarities go far below the surface. Dawson and colleagues have linked the disease in monkeys to the disease in humans at a cellular level. They are the first to discover a chromosome region that is common to macular degeneration in humans and monkeys. The next step is to find the specific genes within the chromosome structure involved with macular degeneration, thus finding possible targets for gene therapy. “If the disease is to be treated, a model animal becomes very valuable,” Dawson said. “Beside, monkeys are easier to test and are always there to keep appointments. They can stand in for people to work out the details of prevention and cure of the disease.” It’s easy to grasp the concept of injecting a healthy gene to replace a broken one in a closed system like the eyeball, but the actual task is not so easily done. Sue Semple-Rowland, an associate professor of neuroscience with UF’s McKnight Brain Institute, discovered, characterized and recently developed a viral therapy to treat the malfunctioning gene that causes vision loss in a type of chicken that is always born blind. The condition is very similar to the blindness experienced by people with Leber congenital amaurosis type 1, or LCA1, yet another hereditary form of vision loss. Chickens, like people but unlike mice, observe colors and function best in the daylight. In fact, these animals have the same highly detailed retinal structure as primates, making them excellent models for testing vision treatments. But the experimental challenges are enormous. First, scientists build a virus capable of delivering the corrective gene to the retina — this one a lentivirus. Then the virus is delivered to a chicken embryo’s developing nervous system through a tiny hole in the shell of an egg. Finally, the egg containing the embryo is nurtured to hatching to produce a live chick, a process requiring precise measures of temperature, humidity and careful handling. Years of trial and error have gone into perfecting this process. The result: Chickens born without sight can see. “We can do amazing things in animal models,” Rowland said, “but this work can’t be done quickly. That’s the hardest thing — knowing there are people who need these treatments now. But we work as fast as we can. You’ll see the first treatments for some of these genetic eye diseases soon, especially after the groundwork for an approved therapy is laid and the therapy works.” But some hereditary eye diseases, such as LHON, which Josh Henderson copes with, pose even greater treatment challenges.
Mitochondria MessLHON is caused by degeneration of the optic nerve. Unfortunately, the genes that cause this degeneration are in the mitochondria, structures within cells that control energy production and other specialized tasks. The reasons why mutations within mitochondrial DNA affects the optic nerve are not only uncertain but investigation has been hampered by lack of an animal model of the disease.
In addition, they’ve shed light on a potential culprit in LHON blindness. They believe harmful free radicals set loose by mutations in mitochondrial DNA are damaging the optic nerve. Free radicals are molecules that have been knocked off balance because they’ve gained an electron. They create a cascade of cellular damage as they try to stabilize themselves by giving electrons to nearby molecules. UF findings suggest that cellular antioxidants — enzymes that mop up free radicals — may become the first line of treatment for a blinding diseases like LHON. In the meantime, Josh Henderson isn’t waiting for a cure to live his life. He joined the wrestling club at Santa Fe Community College and, for a career, is considering following in his father’s footsteps and studying to be a chiropractor. “I’m very hopeful and optimistic,” Henderson said. “But at the same time, I have to be realistic.” William W. Dawson William Hauswirth Susan Semple-Rowland |