|
|
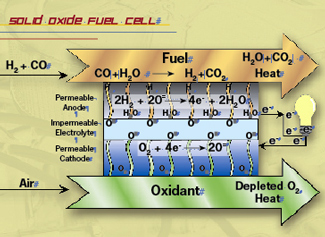
 UF researchers are developing solid oxide fuel cells that take the bang out of drivingBy Joseph Kays If you think $4 a gallon for gas is ridiculous, consider that only about 7 percent, or 28 cents, of that money is actually going toward moving your car. To move the pistons at the heart of your car’s engine, gasoline is oxidized in thousands of little explosions every minute. In the process, more than 62 percent of the fuel’s energy is lost in heat from those explosions. Another 17 percent is lost to idling, 12 percent to driveline friction and braking, and the rest to all those belts and pulleys that run the electrical system. An internal combustion engine converts fuel to energy by burning the fuel, and when there was a seemingly unlimited supply of fuel, this was an economically feasible approach. But as fossil fuel supplies dwindle, demand is growing for ever-more-efficient automobiles. So instead of burning fuel to create energy, researchers like Eric Wachsman are pursuing fuel cells — 21st-century cousins of the battery that exploit electrochemical reactions to create electricity. As a California teenager with a passion for cars, Wachsman could see the future of gas-powered automobiles in the long lines of the 1970s oil crisis. “During the first oil crisis, it was obvious to me that this problem wasn’t going to go away, and that we were going to become more and more dependent on foreign oil,” says Wachsman, a professor of materials science and engineering who has been at UF since 1997. Wachsman also saw firsthand what auto emissions were doing to the environment. “When I was growing up in California,” he says, “the smog around Los Angeles was so bad there was no such thing as a clear day.” Instead of burning, fuel cells electrochemically oxidize the fuel, harnessing the energy released as electrons migrate from one side of the fuel cell to the other. The trick is to get the perfect mix of materials to maximize this game of electron musical chairs. Fuel cells are built like a sandwich, with two porous outer layers called the cathode and the anode. These are separated by a dense, gas-tight middle layer called the electrolyte. Oxygen from air flows through the cathode and the fuel flows through the anode. The oxygen is “reduced” in the cathode, consuming electrons, and the fuel is “oxidized” in the anode, creating electrons. Depending on the type of fuel cell, charged atoms called ions are created and migrate through the electrolyte. This reaction generates electrons, which flow from the anode back to the cathode in an electrical circuit. Typically, a millimeter-thick fuel cell generates about 1 volt, and for a surface area of two square centimeters about 1 Amp of current. So 100 of these 2 cm2 cells assembled in a stack about the size of a pack of gum generates enough power (volts x amps) to light a 100-watt light bulb. Wachsman says fuel cell cars would use far less fossil fuels and produce far less pollutants. “Fuel cells are three times more efficient than an internal combustion engine,” he says. “This high efficiency means you not only get three times the gas mileage, but produce just a third of the carbon dioxide emissions.” Historically, fuel cells have used hydrogen as the fuel, but because hydrogen is difficult to handle and there is no national distribution system for it, solid oxide fuel cells, or SOFCs, are gaining attention. “Rather than forming a hydrogen ion on the fuel side that migrates to the air side, with an SOFC you form an oxygen ion on the air side that migrates to the fuel side,” Wachsman says. “This oxygen ion will then react with any fuel, from natural gas to gasoline and alcohol, and is not limited to just hydrogen.” The challenge with SOFCs is that they require a much higher operating temperature than their hydrogen cousins, known as proton exchange membrane fuel cells, or PEMs.
“Back in the 1990s, when the automobile companies were looking at fuel cells for transportation applications, they focused on PEMs because it would have taken too long for a solid-oxide fuel cell to warm up,” Wachsman says. “You want to turn the key and go, but at those high temperatures you would be sitting in your driveway for hours.” But as automakers moved toward hybrid vehicles that have both an internal combustion engine and a battery, the temperature issue became less important, he says. “With the battery in a hybrid, the fuel cell doesn’t have to operate at the same time as the car,” Wachsman says. “It can warm up while you’re moving and continue to charge the battery after you get to where you’re going.” Wachsman and his colleagues have been experimenting with new materials for the electrolyte, and have succeeded in bringing the operating temperature of their SOFCs down from about 1000º Celsius to less than 500º C. By lowering the temperature, Wachsman says this new generation of fuel cell can be made of less expensive materials that would lead to lower manufacturing costs. Now, Wachsman says, not only are stationary power generating companies like Siemens developing SOFCs, but so are automotive suppliers like Delphi. All of the major automobile manufacturers are designing fuel cell vehicles and the federal government has committed more than a billion dollars to advance the technology. “Fuel cells have application across the spectrum, from cars to stationary power plants,” Wachsman says. “What makes solid oxide fuel cells unique is their fuel flexibility.” And it is that flexibility, he says, that will help bridge the gap between the fossil fuel economy of the present and a hydrogen economy of the future. “In a hydrogen-based system, you’ve got to store hydrogen and that would require a whole new transportation infrastructure,” he says. “The cost to convert every gas station in the U.S. to dispense a gaseous hydrogen instead of a liquid fuel is a major impediment.” With more than $4 million in funding from the U.S. Department of Energy, Wachsman’s SOFC program at UF is considered to be one of the preeminent research programs in the country. “Eric’s research has contributed significantly to advancing the solid oxide fuel cell technology through understanding of the underlying principles, development of high-conductivity electrolytes, and characterization of electrode microstructures,” says Subhash C. Singhal, director of the fuel cell program at the Pacific Northwest National Laboratory in Richland, Washington. “He has also educated a large number of students in this field which bodes well for the future of the fuel cell industry in the United States.” Last fall, the Florida Board of Governors awarded UF a $4.5 million Center of Excellence that includes an incubator where Wachsman and his colleagues can build actual fuel cell stacks. “We already have the science and technology, we’ve just been waiting for a facility to put it all together,” Wachsman says. “Our goal is to take research results in the laboratory and bring them to the prototype or proof-of-concept level. That’s what we need to get industry interested in commercializing this research, so that these clean, efficient technologies can have a real impact on our use of energy.” Eric Wachsman Related Web site: |